Belief BioMed’s AAV-based Gene Therapy Drug Candidate Receives China CDE’s Breakthrough Therapy Designation
2022-08-24
Shanghai, CN, Orange, NC August 24, 2022
Belief BioMed (BBM, Company) announced today, that China Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) has granted Breakthrough Therapy Designation (BTD) to the BBM-H901 injection, the AAV vector gene therapy drug candidate for elimination of bleeding in adult male patients with hemophilia B, which was independently developed and manufactured by Belief BioMed (from its wholly owned subsidiary, Shanghai Belief-Delivery BioMed Co., LTD.).
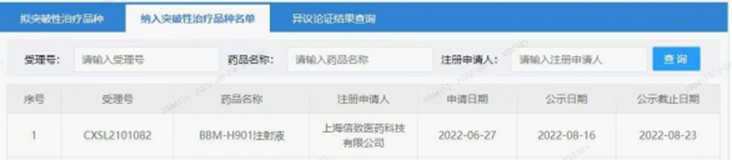
Source: CDE website
BTD is aiming to accelerate drug development for critical diseases and conditions. A drug candidate or treatment qualifies as a breakthrough therapy when it proves in early phase clinical trials substantially improved efficacy or safety over existing drugs or treatments. Drug candidates with the BTD will receive accelerated evaluation by CDE, and opportunities for more intensive CDE guidance and discussions which may lead to fast-track approval when submitted as a New Drug Application (NDA), thereby addressing the unmet patient needs earlier.

Dr. Xiao Xiao, Co-founder, Chairman and Chief Science Officer of BBM
“The Breakthrough Therapy Designation is a significant milestone in the development of BBM-H901 injection, which is the acknowledgement of the product’s safety and efficacy. The Breakthrough Therapy Designation will enable the acceleration of the development of BBM-H901 for the treatment of Hemophilia B, thereby bringing this life-changing innovative drugs to patients sooner,” said Dr. Xiao Xiao, Co-founder, Chairman and Chief Science Officer of Belief BioMed. “Leveraging company's AAV vector innovative technologies, following our guiding principle of "believing in sciences, with patients in our mind", we are fully committed to providing more effective next-generation therapeutics for genetic diseases, age-related degenerative diseases and certain malignant diseases, making gene therapy available and affordable across the world”.
About BBM-H901
BBM-H901 is a cutting-edge bio-engineered adeno-associated viral (AAV) vector utilizing a novel recombinant AAV capsid, containing a codon-optimized human factor IX gene under the control of a liver-specific promoter. Belief BioMed owns the proprietary patents of the capsid and the cassette. Leveraging the serum-free production and chromatographic purification process developed in house by Belief BioMed to produce drugs in line with cGMP requirements, BBM-H901 has demonstrated high efficacy and safety with the FIX (Factor IX) well-expressed in patient’s plasma, achieving “one-single treatment, long-term benefit” effect.
BBM-H901 is one of the first gene therapeutical candidates entering clinical trials in China. As the Investigator Initiated Trial (NCT04135300) was started in 2019, currently the candidate drug development is in the registrational clinical trials (CTR20212816). It is the first AAV gene therapy candidate drug approved to enter registrational clinical trials in China for treatment of hemophilia, and the first intravenous gene therapy for treatment of rare diseases in China and across Asia region. So far, no AAV vector gene therapy drug has been approved for marketing in China.
About Hemophilia
Hemophilia, an inherited bleeding disorder, is mainly caused by mutations in the coagulation factor VIII or IX genes. Spontaneous bleeding may occur due to a significant reduction in coagulation factor VIII activity (FVIII:C, hemophilia A) or factor IX activity (FIX:C, hemophilia B) in patients. Repeated bleeding in joints and muscles may cause lifelong disability. Nowadays, prophylaxis and on-demand therapy with coagulation factor remain the standard clinical treatment for hemophilia. Patients need repeated injections of plasma-derived or recombinant coagulation factors throughout their life to maintain their coagulation function. As a result, the development of drugs that can cure hemophilia is a goal that scientists relentlessly pursue worldwide, and gene therapy has become a cutting-edge technology to cure Hemophilia.
About Belief BioMed
Belief BioMed is aiming to become a globally leading gene therapy company by being committed to providing innovative therapies with improved efficacy for monogenic disorder diseases, age-related degenerative diseases, and certain malignant diseases through its AAV vector technology from early discovery to commercialization. BBM has the proprietary rights of the AAV capsid and the expression cassette and has achieved several proprietary improvements in process and quality control, forming an advanced, reliable, efficient manufacturing process, with a single production cell culture volume of up to 500L currently. The R&D and production strengths of Belief BioMed have been recognized by top investment institutions and enterprises. Belief BioMed has offices, research centers and manufacturing facilities in Shanghai, Hong Kong, Beijing and Suzhou China and North Carolina US.





 Previous
Previous