The Case Report of Belief BioMed’s Hemophilia B Gene Therapy Drug Candidate BBM-H901 Published in The New England Journal of Medicine
2022-10-27
Shanghai, China; Orange, North Carolina, USA 27th October 2022
A case report of Total Knee Arthroplasty after Gene Therapy for Hemophilia B (HB) is published in the world’s leading medical journal, The New England Journal of Medicine, via correspondence, reporting the world’s first total knee arthroplasty (TKA) after a single dose intravenously delivered gene therapy with BBM-H901 for Hemophilia B.
 Fig. 1
Fig. 1
The case reports the world’s first HB patient who received gene transfer with BBM-H901 and subsequently underwent unilateral TKA without exogenous FIX infusion during the perioperative period. An excellent haemostatic effect of gene therapy for the HB patient who underwent major surgery was observed for the first time, demonstrating the repeated success of gene therapy in HB. This case provides a reliable clinical basis for perioperative treatment plans for HB patients who need surgeries after having been treated with gene therapy.
BBM-H901 is a gene therapy for Hemophilia B developed and manufactured in-house by Belief BioMed, which utilizes a liver-tropic adeno-associated viral (AAV) vector carrying cassette coding hyperactive mutant FIX-Padua. The investigator-initiated clinical study includes ten patients with moderate to severe Hemophilia B (FIX: C<2%) who were intravenously infused with 5×1012vg/kg, including the patient in the case reported. BBM-H901 has brought significant clinical benefits to patients during at least a 1.5-year follow-up interval, on average, after treatment. Before the operation, the patient’s transgene-derived FIX: C ranged from 51.4 IU/dL to 85 IU/dL measured by the one-stage method with Dade Actin FSL on a Sysmex CS5100 (Siemens) (Fig. 2). No vector-related adverse effect, no bleeding event, absence of target joints, and no need for FIX replacement therapy after gene therapy during the follow-up interval.
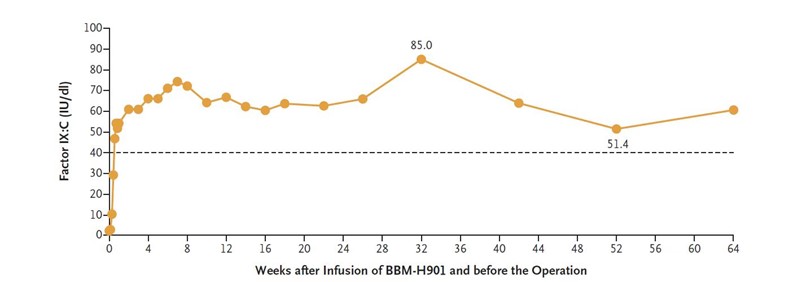 Fig. 2
Fig. 2
This patient’s haemophilia arthropathy, which existed before the gene therapy treatment, progressed and compromised his quality of life. After consulting with a multidisciplinary team, the patient underwent a right TKA successfully. During the perioperative period and without any FIX concentrate infusion, no excessive bleeding was observed during and after surgery – the intraoperative blood loss was estimated at 150 ML. FIX: C was 50.1 IU/dL in the morning of the operation and decreased to 46 IU/dL after the operation on the same day. One day after the procedure, FIX: C rose to 53.8 IU/dL. The upwards trend of FIX: C was observed from D1 to D5 after the operation, and the peak FIX: C was 79.3 IU/dL. Then, the FIX: C decreased gradually to a stable level of approximately 60 IU/dL (Fig. 3).
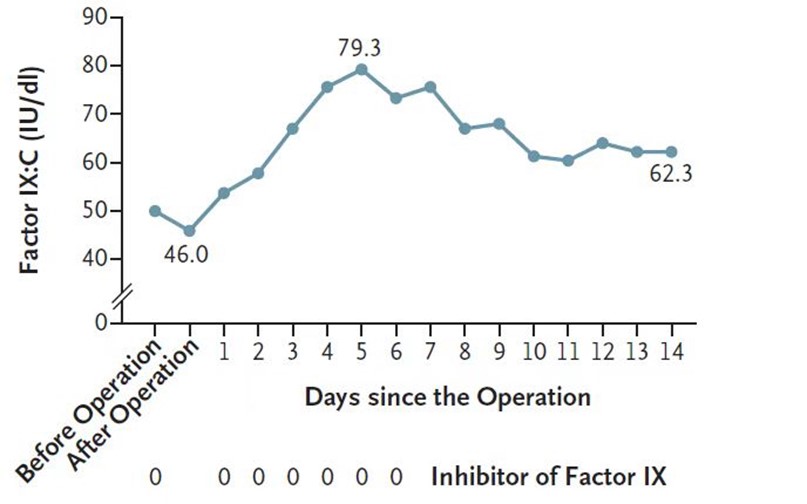
Fig. 3
Overall, the hemostasis effect of gene therapy with BBM-H901 in the setting of major haemostatic challenges in major surgery was verified for the first time in vivo. The safety and efficacy of BBM-H901 are well demonstrated, which could deliver a solution for the long-term treatment of hemophilia B and alleviate the occurrence of related complications for the betterment of patients. The clinical research of BBM-H901 is expected to provide a reliable basis and theoretical support for the clinical application of systematically administered gene therapy drugs in the future and is of important guiding significance for promoting the research and development and clinical translation of gene therapy drugs.
"We’re beyond proud to be able to help this patient. It’s the difference between pure laboratory research and drug development, where the former you can try any novel ideas, but the latter delivers real benefits to patients,” said Dr Xiao Xiao, Chief Scientific Officer and Chairman of Belief BioMed. “We will continue to advance the discovery and research of our gene therapy portfolio across distinct therapeutic areas, to accelerate clinical trials and commercialization of the therapies. We hope gene therapy could be accessible to more patients with rare or common diseases as soon as possible.”
The case report is contributed by the Institute of Hematology and Blood Diseases Hospital at the Chinese Academy of Medical Sciences, Belief BioMed, Shandong Provincial Qianfoshan Hospital, Blood Center of Shandong Province, and University of Pennsylvania School of Medicine, Philadelphia, USA.
About BBM-H901
BBM-H901 is a cutting-edge bio-engineered adeno-associated viral (AAV) vector utilizing a novel recombinant AAV capsid, containing a codon-optimized human factor IX gene under the control of a liver-specific promoter. Belief BioMed owns the proprietary patents of the capsid and the cassette. Leveraging the serum-free production and chromatographic purification process developed in-house by Belief BioMed to produce drugs in line with cGMP requirements, BBM-H901 has demonstrated high efficacy and safety with the FIX (Factor IX) well-expressed in patient’s plasma, achieving “one-single treatment, long-term benefit” effect.
BBM-H901 is one of the first gene therapeutical candidates entering clinical trials in China. The Investigator Initiated Trial (NCT04135300) was started in 2019, and the candidate drug development is currently in the registrational clinical trials (CTR20212816). It is the first AAV gene therapy candidate drug approved to enter registrational clinical trials in China for the treatment of hemophilia, and the first intravenous gene therapy for the treatment of rare diseases in China and across Asia. So far, no AAV vector gene therapy drug has yet been approved for marketing in China.
About Hemophilia
Hemophilia, an inherited bleeding disorder, is mainly caused by mutations in the coagulation factor VIII or IX genes. Spontaneous bleeding may occur due to a significant reduction in coagulation factor VIII activity (FVIII: C, hemophilia A) or factor IX activity (FIX: C, hemophilia B) in patients. Repeated bleeding in joints and muscles may cause lifelong disability, such as hemophilic arthropathy. Nowadays, prophylaxis and on-demand therapy with coagulation factors remain the standard clinical treatment for hemophilia. Patients need repeated injections of plasma-derived or recombinant coagulation factors throughout their life to maintain their coagulation function. As a result, the development of drugs that can cure hemophilia is a goal that scientists relentlessly pursue worldwide, and gene therapy has become a cutting-edge technology to cure hemophilia.
About Belief BioMed
Founded in 2018, Belief BioMed has become a globally leading company by being committed to providing innovative therapies with improved efficacy for monogenic disorder diseases, age-related degenerative diseases and certain malignant diseases through its AAV vector technology from early discovery to commercialization. The R&D and production strengths of Belief BioMed have been recognized by top investment institutions and enterprises. Belief BioMed has offices, research centers and manufacturing facilities in Shanghai, Hong Kong, Beijing and Suzhou China and North Carolina, USA.





 Previous
Previous